

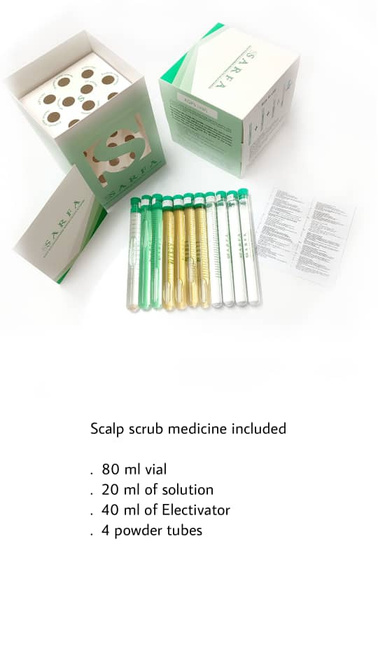
FACT SHEET
As of October, 2022

We’re an agile pharmaceutical company dedicated to simplifying access to life-changing medicine, for good. We provide easy access to fairly-priced medicines through robust manufacturing, consistent supply, and rapid response to your needs, so you can spend your time focusing on your patients.

Based in Chicago, IL
Global network of FDA approved manufacturing
and R&D partners in Europe, India and China
Strategic partnerships with proven track record of
successfully taking new drugs to market
Suite of capabilities with robust manufacturing and R&D Experienced leadership team with strong quality and
compliance expertise overseeing 75 employees
Deep acute channel expertise and customer
relationships, with hospital-based sales force
A BLUEPRINT FOR SUCCESS
•
•
Meitheal founded
• Organization starts to scale
2 products launched
• To further Meitheal growth, $95M investment from NKF • FDA approval of Enoxaparin
• 10 new products launched
•
•
•
Invested in mAb platform Expanded into retail segment 10 new product launches
•
•
•
Advance mRNA candidate in Phase 1 Planned 20+ product launches
Evolve into a biopharmaceuitcal
organization

2017
2018
2019
2020
2021
2022
2023
•
•
•
1st Major GPO Award
FDA approval of Heparin
3 new product launches
• Establishes strategic customer relationships
• Partnered with Premier on Covid-19 research • 11 new product launches
• Achieved ~$100M in sales and profitable
• ~200M in sales
15 new product launches
Established Specialty
Biopharma Commercial
Team
•
•
• Establish Women’s
Fertility Initiative
• Advancing
mAB into clinical
development

2021-22 REVENUE
250
200
US $M
150
Meitheal continues to invest in further developing and expanding each therapeutic category
APPROXIMATELY 50% INCREASE IN PORTFOLIO SIZE OVER NEXT THREE YEARS*
48
2022 NUMBER OF PRODUCTS
17
18
6
7
2025 NUMBER OF PRODUCTS
31
22
11
7
Critical Care
Oncology
Anesthesia
Anti-Infectives
71
100
50
0
2017
2018
2019
2020
2021
2022E
(*based on number of molecules in MH portfolio)
Achieved over
%MARKET
SHARE
15
for 50% of our market launched molecules
(based on IQVIA September 2022 data)
Achieved
LEADING
or SECOND PLACE MARKET SHARE on
(based on IQVIA unit volume September 2022)
10
molecules
OUR PURPOSE
OUR VISION
OUR MISSION
OUR
VALUE PROPOSITION
Simplify access
to life-changing medicine, for good.
Healthcare solutions within reach.
We solve healthcare challenges by building authentic relationships, providing innovative, value-based solutions, and keeping our promises.
Spend more time focusing on your patients. With Meitheal as your partner, you have access to fairly priced, essential medicines — and peace of mind.
www.meithealpharma.com
The brand names mentioned here are the trademarks of their respective owners.
©2022 Meitheal Pharmaceuticals. All rights reserved.
AD22-043

Authors: Grierson, Claire, Nielsen, Erik, Ketelaarc, Tijs, and Schiefelbein, John
Source: The Arabidopsis Book, 2014(12)
Published By: The American Society of Plant Biologists
URL: https://doi.org/10.1199/tab.0172
BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses.
Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use.
Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder.
BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research.
Downloaded From: https://bioone.org/journals/The-Arabidopsis-Book on 02 Dec 2023 Terms of Use: https://bioone.org/terms-of-use
The Arabidopsis Book
© 2014 American Society of Plant Biologists
First published on June 25, 2014: e0172. doi: 10.1199/tab.0172
This is an updated version of a chapter originally published on April 4, 2002, e0060. doi:10.1199/tab.0060
Root Hairs
Claire Griersona, Erik Nielsenb, Tijs Ketelaarc, and John Schiefelbein
d,1
a
School of Biological Sciences, University of Bristol, Bristol, UK BS8 1UG.
b
Department of Molecular, Cellular, and Developmental Biology, University of Michigan, Ann Arbor, MI, USA 48109.
c
Laboratory of Cell Biology, Wageningen University, 6708 PB Wageningen, The Netherlands.
d
Department of Molecular, Cellular, and Developmental Biology, University of Michigan, Ann Arbor, MI, USA 48109.
1
Address correspondence to schiefel@umich.edu
Roots hairs are cylindrical extensions of root epidermal cells that are important for acquisition of nutrients, microbe interac- tions, and plant anchorage. The molecular mechanisms involved in the specification, differentiation, and physiology of root hairs in Arabidopsis are reviewed here. Root hair specification in Arabidopsis is determined by position-dependent signaling and molecular feedback loops causing differential accumulation of a WD-bHLH-Myb transcriptional complex. The initiation of root hairs is dependent on the RHD6 bHLH gene family and auxin to define the site of outgrowth. Root hair elongation relies on polarized cell expansion at the growing tip, which involves multiple integrated processes including cell secretion, endo- membrane trafficking, cytoskeletal organization, and cell wall modifications. The study of root hair biology in Arabidopsis has provided a model cell type for insights into many aspects of plant development and cell biology.
INTRODUCTION
Root hairs are long tubular-shaped outgrowths from root epider- mal cells. In Arabidopsis, root hairs are approximately 10 μm in diameter and can grow to be 1 mm or more in length (Figure 1). Because they vastly increase the root surface area and effec- tively increase the root diameter, root hairs are generally thought to aid plants in nutrient acquisition, anchorage, and microbe inter- actions (Hofer, 1991).
Root hairs in Arabidopsis have attracted a great deal of at- tention from plant biologists because they provide numerous advantages for basic studies of development, cell biology, and physiology (Schiefelbein and Somerville, 1990). The presence of root hairs at the surface of the root and away from the plant body means that they are easily visualized and accessible to a variety of experimental manipulations. Further, the lack of a cu- ticle layer allows physical and chemical probes to be applied with ease. Root hairs grow rapidly, at a rate of more than 1 μm/min, which facilitates studies of cell expansion. Perhaps most impor- tantly, root hairs are not essential for plant viability, which permits the recovery and analysis of all types of mutants that alter root hair development and function. Also, root hairs become visible on seedling roots shortly after seed germination, which enables genetic screens and physiological tests to be performed rapidly with large numbers of individuals grown on defined media in Petri dishes (Figure 2). Finally, the development of root hairs (and their

Figure 1. Scanning electron micrograph of a root hair cell. The hair pro- duced by this cell is approximately 1/3 of its final length.
resident epidermal cells) occurs in a predictable and progressive manner in cells organized in files emanating from the root tip (Fig- ure 3). This provides the opportunity for detailed analysis of the cellular changes that occur during the entire process of root hair formation.
Downloaded From: https://bioone.org/journals/The-Arabidopsis-Book on 02 Dec 2023 Terms of Use: https://bioone.org/terms-of-use
Report
Stimulation of Hair Growth by Small Molecules that Activate Autophagy
Graphical Abstract








Authors
Min Chai, Meisheng Jiang,
Laurent Vergnes, ..., Gay M. Crooks, Karen Reue, Jing Huang
Corresponden ce
In Brief
Hair regeneration requires reactivating dormant hair follicle stem cells. Chai et al. discover that pharmacological induction of autophagy is sufficient to activate quiescent telogen hair follicles, initiating new anagen hair growth. Hair loss resulting from shortened anagen, lengthened telogen, and/or impeded anagen induction may thereby be
rescued by activating autophagy.
Highlights
d
mTOR and AMPK modulation by rapamycin, metformin, and a-KG induces anagen hair growth
Autophagy induction is necessary and sufficient for anagen
entry and hair growth
Autophagy is increased during anagen phase of the natural
hair follicle cycle
Aged mice fed the autophagy-inducing metabolite a-KB are
protected from hair loss
d
d
d
27
Chaietal.,2019,CellReports ,3413–3421 June 18, 2019 ª 2019 The Authors. https://doi.org/10.1016/j.celrep.2019.05.070
Current Biology Vol 23 No 2 R52
and take over the discipline. If we
want to make sure that the biology
of the future preserves our hard-won biological perspectives, knowledge and insights, we need to be able to
do the analyses and deal with all
these data ourselves.
For young scientists embarking
on a PhD, make sure your PhD topic
is something you love, and that
your question is one whose answer you care deeply about. Don’t settle
for less than this, or you’ll lack the drive needed to work to your own
full potential. And if there’s some
body of knowledge or theory that is important to your question, whatever the discipline, just roll up your
sleeves and learn it. For post-docs, one word: ‘publish!’ And don’t spend months trying to get a paper perfect in every detail before submitting: your reviewers will find flaws no matter what. Spend your time getting the experiments and analysis right, not perfecting the writing.
Your website says you play music and paint: does science influence your creative work? While in college
I seriously considered a career in the arts, and many of my closest friends and band-mates went on to become professional musicians. I’ve played in rock and salsa bands and an African drumming ensemble, and I still play
a lot of guitar and write and record songs. And recently, we’ve been studying the biology and evolution of music, and I’ve really been enjoying the opportunity to combine music
with scientific research.
Regarding the influence of
science, I draw the figures for a lot
of my publications (ink drawings or watercolors, reworked with Adobe Illustrator). I’ve also written some biological songs, including “I Don’t Believe in Evolution”, which pokes
fun at creationists and has been live- broadcast on Italian radio (and even served as a ring tone on some of my students’ phones!).
But frankly, I’m happy to have
science as my ‘day job’ and music and painting as hobbies: I think the pressure to make money with art would take the fun out of it.
So you’re glad you became a scientist? Absolutely. I feel incredibly fortunate to be a scientist. Sure, scientists’ salaries are not usually commensurate to their education
and ability. But how many people are lucky enough to be paid to follow
their interests and satisfy their own curiosity every day?
What are the most exciting topics you are researching right now?
At the moment I’m very excited
about our new research program
in empirical aesthetics, trying to understand the biological roots of the visual arts, and in particular of the human love for symmetry and order. Humans around the planet surround themselves with decorative patterns, with no obvious function, such as weaving, quilting, decorated pottery, clothes, tattoos and architectural ornament. Oddly, art historians have largely focused on representational
art by great geniuses, and neglected this much more widespread, popular and presumably ancient form of art (often relegated to ‘craft’). We’ve
been bringing ordinary people into
the lab and studying the kinds of patterns they make using computer interfaces (as well as what they like, and what kind of rules they can perceive). It looks like there is a deep biological drive in our species — what the art historian Ernst Gombrich
called our ‘sense of order’ — that hasn’t received enough attention.
I’m also very excited about our
work in bioacoustics, trying to understand how animals produce
their sounds. This is a truly inter- disciplinary bridging area, spanning
an amazing breadth of disciplines
from physics to physiology to
behavior, cognition, and evolution. It also relies on comparative anatomy, so you get to dig out old anatomical papers documenting weird and wonderful adaptations for sound production that were forgotten long ago, and then try to understand
them from the viewpoint of modern acoustics and nonlinear dynamics. We’re studying vocal production in alligators, deer, primates, ravens, parrots, and lots of other species,
but it is amazing how the same physical phenomena and principles (mostly originally discovered in
human speech) seem to underlie
all this diversity. It’s a comparative biologist’s dream come true.
Quick guide
Aquaporins
A.S. Verkman
What are aquaporins? Aquaporins (often called aquaporin water
channels) are a family of small,
integral membrane proteins that are expressed broadly throughout the animal and plant kingdoms. They
have a similar basic structure, with aquaporin monomers consisting of
six transmembrane helical segments and two short helical segments that surround cytoplasmic and extracellular vestibules connected by a narrow aqueous pore (Figure 1A). They contain several conserved motifs, including NPA sequences in their short helical segments. Aquaporin monomers assemble as tetramers in membranes, with each monomer functioning independently. Some aquaporins,
such as mammalian AQP4, can further aggregate in cell membranes to form supramolecular crystalline assemblies called orthogonal arrays of particles.
What do aquaporins do at the
molecular level? The primary function
of most aquaporins is to transport
water across cell membranes in
response to osmotic gradients created
by active solute transport. Because the
water transport capacity of aquaporin
monomers is low, membranes often
contain a high density of aquaporins,
up to 10,000 per square micron,
to increase water permeability
substantially above that in the absence
of aquaporins. Molecular dynamics
simulations suggest that steric
factors and electrostatic interactions
in the aqueous pore are responsible
for the selectivity of aquaporins for
water. A subset of aquaporins, called
aquaglyceroporins also transport
glycerol. The pore diameter of the
aquaglyceroporins is slightly greater
than that of the water-selective
aquaporins, and the pore is lined
by relatively hydrophobic residues
compared with the pore of a water-
selective aquaporin. In addition to
water and glycerol, there is evidence,
some of which is controversial, that
some aquaporins pass gases (CO2,
NH3, NO, O2), various small solutes
such as H2O2 and arsenite, and
Department of Cognitive Biology, Faculty of Life Sciences, University of Vienna, 14 Althanstrasse, A-1090 Vienna, Austria. E-mail: tecumseh.fitch@univie.ac.at



2 of 25
The Arabidopsis Book

Figure 2. Development of Arabidopsis seedlings growing on agarose-so- lidified nutrient medium in vertically-oriented Petri plates. The roots grow along the surface of the medium, and root hairs are visualized easily using a low-magnification microscope.
This chapter provides a summary of the development, struc- ture, and function of root hairs in Arabidopsis. Particular empha- sis is placed on recent findings using molecular genetics to ex- plore root hair development. Recent reviews emphasizing varied aspects of Arabidopsis root hairs have been published (Ishida et al., 2008; Schiefelbein et al., 2009; Tominaga-Wada et al., 2011;
Benitez et al., 2011; Ryu et al., 2013).
ROOT HAIR CELL SPECIFICATION
Pattern of Epidermal Cells in the Root
In Arabidopsis, the epidermal cells that produce root hairs (root hair cells) are interspersed with cells that lack root hairs (non-hair cells). Thus, the first step in root hair development is the specifi- cation of a newly-formed epidermal cell to differentiate as a root hair cell rather than a non-hair cell. This process has been studied intensively during the past several years because it serves as a simple model for understanding the regulation of cell-type pattern- ing in plants.
The Arabidopsis root epidermis is generated from a set of 16 initial (stem) cells that are formed during embryogenesis (Dolan et al., 1993; Scheres et al., 1994; Baum and Rost, 1996; see also the chapter on root development in this book). These initials are termed epidermal/lateral root cap initials because they also give rise to the cells of the lateral root cap (Dolan et al., 1993; Scheres et al., 1994). The immediate epidermal daughter cells produced from these initials undergo secondary transverse divisions in the meristematic region of the root, and these divisions (typically 5 or 6 rounds per daughter cell) serve to generate additional cells within the same file (Baum and Rost, 1996; Berger et al., 1998b). Further- more, anticlinal longitudinal divisions occasionally occur and result in an increase in the number of epidermal cell files; this activity causes the observed number of epidermal cell files in the seedling root to vary from 18 to 22 (Galway et al., 1994; Baum and Rost,


Figure 4. Transverse section of an Arabidopsis root, showing the position- dependent pattern of hair cells and non-hair cells. The hair-bearing cells are located outside the space separating two cortical cells (the H cell posi- tion), whereas the non-hair cells are located outside a single cortical cell (the N cell position). Three hairs are visible in this section; the other cells in the H position possess hairs that are located outside the field of view.
Figure 3. Photograph of a root tip showing the progressive development of root hair cells.
Nature of the Cell Patterning Information
1996; Berger et al., 1998b). The epidermal cells are symplastically connected during much of their development (Duckett et al., 1994). The root epidermis of Arabidopsis, like other members of the family Brassicaceae, possesses a distinct position-dependent pattern of root hair cells and non-hair cells (Cormack, 1935; 1949; Bunning, 1951; Dolan et al., 1994; Galway et al., 1994). Root hair cells are present outside the intercellular space between two underlying cortical cells (i.e., located outside an anticlinal corti- cal cell wall, called the “H” position), whereas non-hair cells are present over a single cortical cell (i.e., located outside a periclinal cortical cell wall, called the “N” position) (Figure 4). Because the Arabidopsis primary root consistently possesses eight files of cor- tical cells, there are eight root-hair cell files and approximately 10 to 14 non-hair cell files (Dolan et al., 1994; Galway et al., 1994). The simple correlation between cell position and cell type differ- entiation implies that cell-cell communication events are critical
for the establishment of cell identity in the root epidermis.
An exception to this pattern exists near the root-hypocotyl junc- tion, in a region containing 3-7 tiers of cells called the collet (Par- sons, 2009). Here, every epidermal cell forms a root-hair-like exten- sion during early seedling growth (Scheres et al., 1994; Lin and Schiefelbein, 2007; Sliwinska et al., 2012). Consistent with this ex- ceptional pattern, genes that specify the non-hair fate are not active in this region (Lin and Schiefelbein, 2007). Interestingly, this region differs from the rest of the root by possessing a second (incomplete) layer of cortical cells (Lin and Schiefelbein, 2007), due to transition from the cellular anatomy of the hypocotyl (two cortical layers) to the root (one cortical layer). Furthermore, the root hairs in the collet arise synchronously, rather than the progressive formation of root
hairs within cell files at the root apex (Sliwinska et al., 2012).
The information that directs the position-dependent epidermal cell pattern is provided at an early stage in epidermis development, because immature epidermal cells destined to become root-hair cells (trichoblasts) can be distinguished from immature non-hair cells (atrichoblasts) prior to hair outgrowth. Specifically, the dif- ferentiating root-hair cells display a greater rate of cell division (Berger et al., 1998b), a reduced cell length (Dolan et al., 1994; Masucci et al., 1996), greater cytoplasmic density (Dolan et al., 1994; Galway et al., 1994), a lower rate of vacuolation (Galway et al., 1994), unique cell surface ornamentation (Dolan et al., 1994), and distinct cell wall epitopes (Freshour et al., 1996).
A more-precise understanding of the timing of the patterning information has been provided by the use of two reporter gene fu- sions, a GLABRA2 (GL2: At1g79840) gene construct (Masucci et al., 1996; Lin and Schiefelbein, 2001) and an enhancer-trap GFP construct (line J2301; Berger et al., 1998c). Each of these report- ers are expressed in the N-cell position (epidermal cells located outside a periclinal cortical cell wall) within the meristematic re- gion of the root (Figure 5). Careful examination using these sensi- tive reporters reveals position-dependent gene expression within, or just one cell beyond, the epidermal/lateral root cap initials, which implies that patterning information may be provided (and cell fates begin to be defined) within these initial cells and/or their immediate daughters (Masucci et al., 1996; Berger et al., 1998a). The presence of differential gene expression in the early meri- stem led to the possibility that the epidermal cell pattern may be initiated during embryogenesis, when the basic root structure and meristem initials are formed (Scheres et al., 1994). Indeed, the analysis of the J2301 enhancer-trap GFP (Berger et al., 1998a) and the GL2::GFP (Lin and Schiefelbein, 2001) reporters show that the epidermal cell specification pattern becomes established
Downloaded From: https://bioone.org/journals/The-Arabidopsis-Book on 02 Dec 2023 Terms of Use: https://bioone.org/terms-of-use

Figure 5. Expression of the GL2::GUS reporter fusion during root devel- opment.
(A) Surface view showing preferential expression in the meristematic re-
gion. Bar = 50 μm.
(B) Transverse section showing preferential expression in the N-cell posi-
tion of the epidermis. Bar = 20 μm.
during embryonic root development in Arabidopsis (Figure 6). The GL2::GFP exhibits the earliest expression, beginning at the early heart stage, which is prior to the formation of the root meristem. For each of these reporters, expression is detected in a position- dependent epidermal pattern that mirrors the post-embryonic pat- tern (Berger et al., 1998a; Lin and Schiefelbein, 2001). Thus, it appears that positional information is provided during embryonic root development and acts to establish the proper pattern of gene activities that ultimately leads to appropriate post-embryonic cell type differentiation.
To determine whether positional information is also provided
to epidermal cells post-embryonically, two sorts of experiments have been conducted. In one, a detailed analysis of peculiar epidermal cell clones was performed (Berger et al., 1998a). The clones examined were ones derived from rare post-embryonic longitudinal divisions of epidermal cells, which causes the two re- sulting daughter cells to occupy different positions relative to the underlying cortical cells. The cells within these clones expressed marker genes and exhibited cellular characteristics that are ap- propriate for their new position (Figure 7). In a second set of ex- periments, specific differentiating epidermal cells were subjected to laser ablation such that neighboring epidermal cells were able

